When it comes to blockbuster drugs in these years, it is necessary to mention the HER2-directed antibody-drug conjugates (ADC) jointly developed and commercialized by Daiichi Sankyo/AstraZeneca: DS-8201 (T-DXd, Trastuzumab Deruxtecan, trade name: Enhertu).
Enhertu consists of a HER2 monoclonal antibody (trastuzumab) attached to a topoisomerase I inhibitor (DXd) payload, an exatecan derivative (DX-8951 derivative, DXd), via a stable tetrapeptide-based cleavable linker.
Advantages of Enhertu
Enhertu belongs to the third generation of ADC drugs. Benefiting from the site-specific conjugation technology, ENHERTU has a homogeneous and high drug-to-antibody ratio (DAR) of ~8 molecules of cytotoxic agent per antibody. Meanwhile, it can exert bystander effect, with significantly enhanced killing effect, especially on cancer cells with low expression of antigens.
Enhertu possesses a high DAR compared to other ADCs, reaching a theoretical maximum of 8 and ensuring homogeneity. Enhertu couples approximately 8 cytotoxic molecules per antibody, which are stabilized in plasma by a unique tetrapeptide linker and can be selectively cleaved by enzymes upregulated in tumor cells, making targeted local delivery of payload possible. Statistically, the number of cytotoxic drugs carried by the marketed ADC antibodies is generally scattered randomly, ranging from 2 to 5. The high DAR allows the efficacy of Enhertu to be considered 2 to 4 times higher than previously approved marketed ADC drugs.
Secondly, Enhertu has the bystander effect. The cleavable tetrapeptide linker releases DXd hydrolysate by the action of lysosomes, which has good lipophilicity and is more favorable to penetrating the cell membrane bilayer phospholipid structure and plays a bystander effect. Thus, regardless of the HER2 expression level of tumor cells, high cell membrane permeability can extend its cytotoxic effect to neighboring tumor cells, and the bystander effect of T-DXd only acts on the tumor microenvironment and does not lead to systemic toxicity.
Enhertu FDA Approval History
In December 2019, the FDA approved the marketing of Enhertu, under the trade name Enhertu, for the treatment of patients with unresectable or metastatic HER2-positive breast cancer who have received treatment with 2 or more anti-HER2 therapies. Since then, Enhertu has received global approval for a variety of indications, including:
- • HER2-positive (IHC 3+ or ISH+) breast cancer (2019, 2022)
- • HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer (2022)
- • HR-positive, HER2-low (IHC 1+ or IHC 2+/ISH-) or ultra-low HER2-expressing (IHC 0) breast cancer (2025)
- • HER2-positive (IHC 3+ or IHC 2+/ISH+) gastric or gastroesophageal junction (GEJ) adenocarcinoma (2021)
- • HER2 (ERBB2) activating mutation-positive non-small cell lung cancer (2022)
- • HER2-positive (IHC 3+) solid tumors (2024)
HR-positive, HER2-low (IHC 1+ or IHC 2+/ISH-) or ultra-low HER2-expressing (IHC 0) breast cancer
On January 27, 2025, Enhertu received approval from the U.S. FDA for the treatment of adult patients with unresectable or metastatic hormone receptor (HR)-positive, HER2-low (IHC 1+ or IHC 2+/ISH-) or HER2-ultralow (IHC 0 with membrane staining) breast cancer. This approval is for patients whose disease has progressed on one or more prior endocrine therapies in the metastatic setting, as determined by an FDA-approved test.
This approval was primarily based on the promising results from the Phase III DESTINY-Breast06 clinical trial. This global, multicenter, randomized, open-label trial (n=866) assessed the efficacy and safety of Enhertu (5.4 mg/kg) compared to standard chemotherapy treatments—capecitabine, paclitaxel, and nab-paclitaxel—in patients with HR-positive, HER2-low or HER2-ultralow metastatic breast cancer who had experienced disease progression after prior endocrine therapy.
The results demonstrated that Enhertu significantly reduced the risk of disease progression or death by 36% compared to chemotherapy. The median progression-free survival (PFS) for patients receiving Enhertu was 13.2 months, compared to just 8.1 months for those receiving chemotherapy. Additionally, the confirmed objective response rate (ORR) for Enhertu was 62.6%, versus 34.4% for chemotherapy. These results provide strong evidence of Enhertu's superior efficacy and safety profile in treating this patient population.
HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer
Enhertu exerts potent antitumor effects in patients with low HER2 expression (IHC1+ or IHC2+/ISH-). Clinical data showed that after co-culture of HER2 high-expressing cells and HER2 negative cells, DS-8201 could kill both HER2 high-expressing cells and HER2 negative cells. On August 6, 2022, the FDA granted accelerated approval of DS-8201 for the treatment of patients with HER2 low-expressing metastatic breast cancer, making it the first HER2-directed therapy to show a survival benefit in HER2-low metastatic breast cancer. This approval is based primarily on data from the phase III trial DESTINY-Breast04, which showed that DS-8201 demonstrated superior progression-free survival and overall survival benefit over chemotherapy. Specifically, in the HR+ and overall populations, the DS-8201 group had significantly better mPFS of 10.1 and 9.9 months and mOS of 23.9 and 23.4 months, respectively, than the control group.
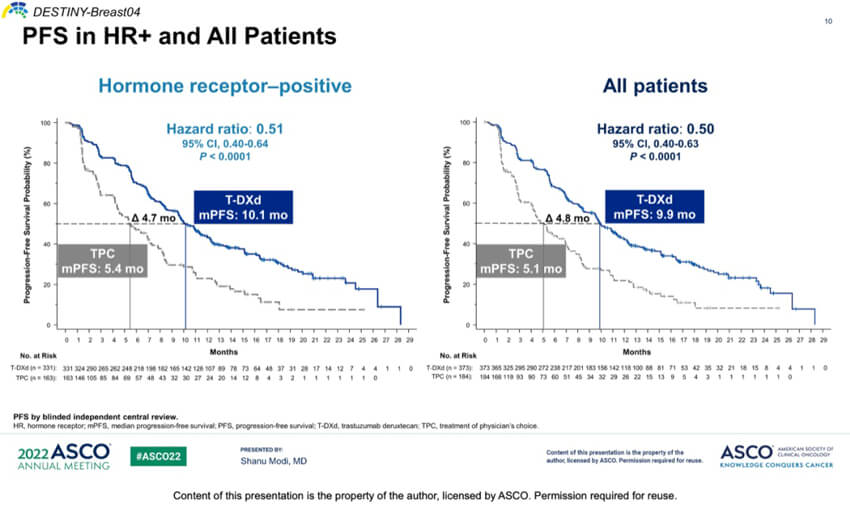
Figure 1. DESTINY-Breast04 PFS in HR+ and all Patients, source: 2022 ASCO
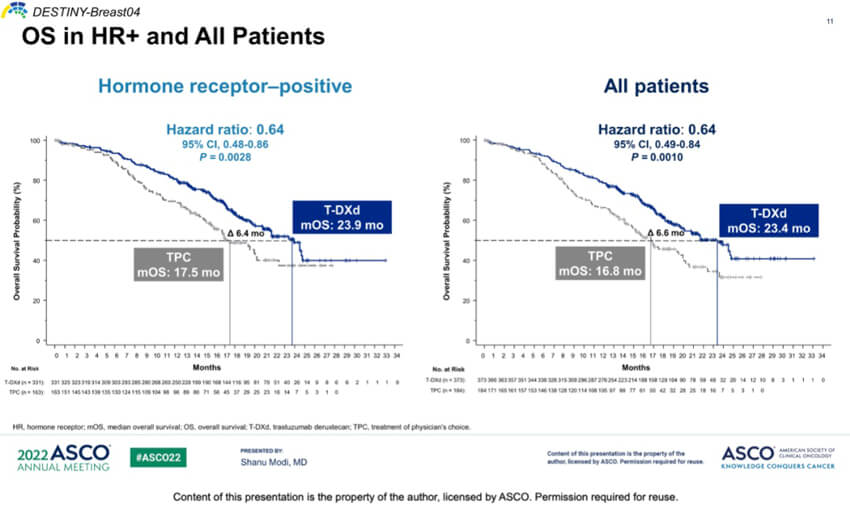
Figure 2. DESTINY-Breast04 OS in HR+ and all Patients, source: 2022 ASCO
Non-Small Cell Lung Cancer
On August 12, 2022, the FDA announced the accelerated approval of an expanded indication for Enhertu for the treatment of patients with unresectable or metastatic non-small cell lung cancer (NSCLC) carrying an activating HER2 mutation. This is the first drug approved by the FDA for the treatment of HER2 mutated NSCLC. The accelerated approval is based on positive results from the multicenter, randomized, double-blind, dose-optimized DESTINY-Lung02 study. In the primary efficacy analysis cohort, which included 52 patients, Enhertu achieved a confirmed objective response rate of 58% (ORR, 95% CI: 43, 71) with a median duration of response of 8.7 months (DOR, 95% CI:7.1, not evaluable).
Gastric or gastroesophageal junction (GEJ) adenocarcinoma
On January 18, 2021, Enhertu was approved by the FDA for the treatment of adult patients with locally advanced or metastatic HER2-positive gastric or gastroesophageal junction (GEJ) adenocarcinoma who have received a prior trastuzumab-based regimen.
This approval was based on the positive results from the Phase II DESTINY-Gastric01 trial. The study demonstrated significant and clinically meaningful improvements in overall survival (OS) and objective response rate (ORR) in patients with advanced gastric cancer or GEJ adenocarcinoma who had previously undergone at least two therapies, including trastuzumab, fluoropyrimidines, and platinum-based chemotherapy.
In this trial, patients treated with Enhertu experienced a 41% reduction in the risk of death compared to those receiving chemotherapy. The pre-specified interim analysis revealed that the median OS for patients on Enhertu was 12.5 months, compared to 8.4 months for those receiving chemotherapy. Additionally, the ORR for Enhertu was 40.5%, while chemotherapy achieved an ORR of just 11.3%. Enhertu also demonstrated a higher rate of tumor response, with 7.9% of patients achieving a complete response and 32.5% achieving a partial response, compared to 0% complete response and 11.3% partial response in the chemotherapy group. These findings underscore the superior efficacy of Enhertu in this patient population.
Enhertu Sales and Prospects
Combined sales of Enhertu, recorded by Daiichi Sankyo and AstraZeneca, amounted to $3,754 million in FY 2024 (FY 2023: $2,566 million). This growth is primarily attributed to its strong performance in the second-line treatment of HER2-positive breast cancer and its expanding indications in HER2-low expressing breast cancer. Enhertu is currently the best-selling ADC drug.
Conclusion
The clinical data of DS-8201 is exceptional. It is not only a highly effective bailout drug for HER2-targeted breast cancer patients, but also expands the benefit population of HER2-targeted therapy (from HER2-positive to low expression), and performs well in the treatment of lung, gastric and intestinal cancers, which is a new dawn for HER2 mutated tumor patients in the future.
Biopharma PEG provides GMP standard PEG derivatives and bulk orders via custom synthesis, offering the opportunity to match customers' special quality requirements. ADC linkers with molecular weights, branching, and functional groups not listed in our online catalog may be available by custom synthesis.
Related Articles:
ADC Drugs For Non-small Cell Lung Cancer
Antibody–Drug Conjugates for the Treatment of Breast Cancer
Global Sales of Antibody-drug Conjugates (ADCs) in 2024
The Bystander Effect of ADCs

