Antibody-Drug Conjugates (ADCs) combine the tumor-targeting specificity of monoclonal antibodies with the potent cytotoxic activity of chemotherapeutic agents (payload). These two components are linked together via a carefully designed chemical linker, allowing for precise targeting while delivering powerful anti-cancer effects, thus improving the therapeutic window of the payload [1]. To date, 17 ADCs have been approved globally, with over hundreds of ADC candidates currently in clinical trials.
However, traditional ADCs often face significant clinical hurdles, such as suboptimal therapeutic efficacy, severe side effects, and the development of drug resistance, which restrict their widespread use. In response to these limitations, a promising next-generation solution—dual-payload ADCs—has emerged. These novel ADCs feature two distinct therapeutic payloads attached to the same antibody, maximizing treatment efficacy through synergistic effects and lowering the likelihood of drug resistance.
Advantages of Dual-Payload ADCs
Dual-payload ADCs have shown significant advantages in cancer treatment due to their unique dual mechanisms and precise delivery capabilities.
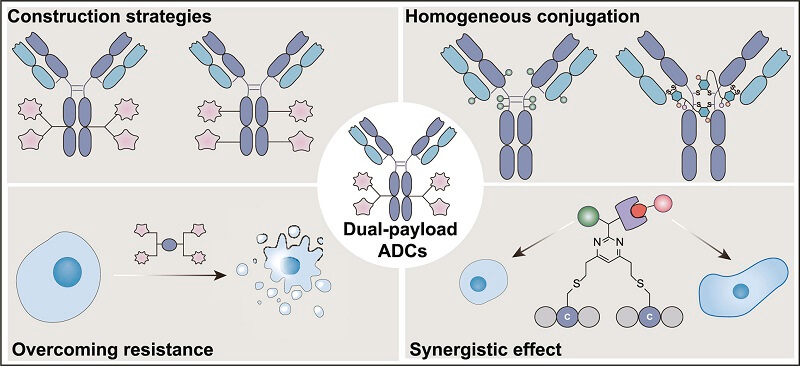
Figure 1. Advantages of Dual-Payload ADCs [2]
Enhanced Therapeutic Efficacy through Synergistic Effects
Dual-payload ADCs can combine different cytotoxic payloads to target various biological pathways or multiple nodes within the same pathway, achieving synergistic effects and potentially reducing toxic side effects. For example, combining topoisomerase inhibitors with PARP inhibitors in a dual-payload ADC can simultaneously act on tumors with homologous recombination repair (HRR) defects and normal homologous recombination, enhancing efficacy. Additionally, the combination of microtubule inhibitors with immunomodulators (such as STING agonists) can directly kill tumor cells while activating antitumor immunity.
Overcoming Drug Resistance through Mechanism Complementarity
Dual-payload ADCs can simultaneously carry two cytotoxic payloads with different mechanisms of action (e.g., DNA damage agents and microtubule inhibitors), targeting multiple key tumor pathways. This dual-target approach significantly reduces the likelihood of resistance. Even if tumor cells develop resistance to one payload, the other payload can still effectively exert its cytotoxic effects, greatly lowering the incidence of resistance. Furthermore, by covering various tumor subtypes, dual-payload ADCs help minimize resistance caused by the loss or mutation of a single target.
Reduced Toxicity through Targeted Delivery and Dose Optimization
Dual-payload ADCs utilize antibody-targeted delivery systems, which allow for precise drug delivery to tumor cells, minimizing toxicity to healthy tissues. The synergistic action of the two payloads means that effective tumor killing can be achieved at lower doses, reducing unnecessary side effects. Additionally, optimizing the drug-to-antibody ratio (such as 2+2 or 4+2 combinations) and enhancing payload stability helps improve efficacy while balancing toxicity and therapeutic benefits.
Bystander Effect
Dual-payload ADCs may also remodel the tumor microenvironment by targeting fibroblasts or immunosuppressive cells within it, through certain payloads (such as RNA interference molecules), further enhancing overall therapeutic effects. This bystander effect not only strengthens tumor killing but may also improve patient immune responses.
Advancements in Dual-Payload ADC Research
Although dual-payload ADCs are still in the early stages of development compared to traditional ADCs, their potential for multi-target, multi-mechanism therapy has been widely recognized, and significant progress has been made.
Introduction of the First Dual-Payload ADC
In 2017, Levengood et al. introduced the concept of dual-payload ADCs, combining two microtubule inhibitors—MMAE and MMAF—linked through an orthogonally protected cysteine residue with a drug-to-antibody ratio (DAR) of 16 (8+8). This innovation marked the initial global recognition of dual-payload ADCs and provided a crucial foundation for subsequent research. The combination demonstrated significant therapeutic effects in xenograft models of large cell lymphoma, highlighting the potential of dual-payload ADCs in overcoming tumor resistance [3].
New Payload Combinations
In 2018, Kumar et al. further expanded the application of dual-payload ADCs by proposing a novel combination of MMAE and pyrrolobenzodiazepine (PBD) dimers, linked to the same antibody site using a heterotrifunctional linker. This strategy showed pronounced in vitro antitumor activity in HER2-expressing MDA-MB-453 cells, offering a new direction for the diversified development of dual-payload ADCs [4].
Flexible DAR Combinations and Structural Optimization
In 2021, Kyoji Tsuchikama’s research team at the University of Texas Health Science Center successfully developed homogeneous dual-payload ADCs using click chemistry methods, significantly enhancing the flexibility of DAR combinations, such as 2+2, 4+2, and 2+4. This advancement allows for better optimization of therapeutic efficacy and toxicity at different drug ratios, while also improving pharmacokinetic properties [5].
The 2025 AACR abstract submissions have revealed promising preclinical presentations on 14 dual-payload ADCs, many of which have never been disclosed before.
| Project | Company | Target | Payloads | Abstract |
| DXC018 | Hangzhou Dac | HER2 x HER2 | Topo1i + antimetabolite inhibitor | 2872 |
| Unnamed | Pinotbio | HER2 | Topo1i + tubulin inhibitor | 6753 |
| CTPH-02 | Celltrion | HER2 | Undisclosed | 6755 |
| Unnamed | MediLink | HER2 | Topo1i + tubulin inhibitor | 1804 |
| IMD526 | Affinity Biopharmaceutical | HER2 | Topo1i + TLR7/8 agonist (for example) | 1805 |
| IMD2126 | PD-L1 | |||
| IMD2113 | EGFR x TROP2 | |||
| JSKN021 | Jiangsu Alphamab | EGFR x HER3 | Topo1i + MMAE | 5451 |
| KHN922 | Chengdu Kanghong | HER3 | Topo1i + RNA pol 2 inhibitor | 1587 |
| KH815 | TROP2 | Topo1i + RNA pol 2 inhibitor | 1586 | |
| Unnamed | Acepodia | GPC3 | Undisclosed | 1785 |
| TJ102 | Phrontline Biopharma | CDH6 x FR伪 | Undisclosed | LB021 |
| Unnamed | Araris | NaPi2b | Topo1i + Topo1i | LB138 |
| Unnamed | Sutro | Undisclosed | Topo1i + PARPi | 2870 |
Table. 2025 AACR abstract of 14 dual-payload ADCs [7]
KH815: The First Clinical Dual-Payload ADC
On March 27, Chengdu Kanghong Biotech announced the approval from the Human Research Ethics Committee in Australia for the Phase I clinical trial application of its injectable KH815. This marks KH815 as the world's first dual-payload ADC to enter clinical trials, drawing significant attention within the industry.
KH815 is a novel dual-payload ADC targeting Trop2. It has the potential to overcome drug resistance by inhibiting tumor cells at both the RNA and DNA levels. Additionally, it reduces P-glycoprotein (P-gp) and HSP70 protein expression, improving chemotherapy sensitivity. KH815 uses a humanized IgG1 antibody (hRS7) to target Trop2 and combines a topoisomerase I inhibitor (TOP1i) and RNA polymerase II inhibitor (RNA POL IIi) with a DAR of 7.5 (4+3.5), ensuring high purity and stability.
In preclinical models, including cell-derived xenograft (CDX) and patient-derived xenograft (PDX) models, KH815 demonstrated dose-dependent tumor growth inhibition. It showed superior antitumor activity compared to single payload TOP1i ADCs at equal or lower doses. Additionally, KH815 outperformed Trodelvy and other TOP1i-ADCs in models pretreated with Trodelvy and in P-gp-expressing HCT-15 cells, confirming its potential to overcome resistance to TOP1i-ADCs.
In non-GLP toxicity studies in cynomolgus monkeys, KH815 exhibited a favorable safety profile with a highest non-severe toxic dose (HNSTD) of 40 mg/kg. Overall, KH815 showed strong preclinical antitumor activity, the ability to overcome resistance, and an acceptable safety profile, supporting its potential as a promising cancer treatment candidate.
Challenges and Prospects
Despite the significant therapeutic potential of dual-payload ADCs, their development faces multiple challenges. First, achieving a balance between efficacy and safety is difficult, as the use of two cytotoxic agents with different mechanisms can lead to complex metabolic pathways and unpredictable toxicity risks. High DAR values may trigger adverse reactions, and antibody modifications could affect stability and immunogenicity. Additionally, the complexity of the manufacturing process, particularly in conjugating two payloads, controlling ratios, and ensuring product uniformity, remains a key technical bottleneck. Nonetheless, with ongoing advancements in technology, dual-payload ADCs are expected to demonstrate even stronger efficacy across various therapeutic combinations in the future.
References:
[1] Fu, Z., et al., Antibody drug conjugate: the "biological missile" for targeted cancer therapy. Signal Transduct Target Ther, 2022. 7(1): p. 93.
[2] Tao J, Gu Y, Zhou W, Wang Y. Dual-payload antibody-drug conjugates: Taking a dual shot. Eur J Med Chem. 2025;281:116995. doi:10.1016/j.ejmech.2024.116995
[3] Levengood, M. R.; Zhang, X.; Hunter, J. H.; Emmerton, K. K.; Miyamoto, J. B.; Lewis, T. S.; Senter, P. D. Orthogonal Cysteine Protection Enables Homogeneous Multi-Drug Antibody-Drug Conjugates. Angew Chem Int Ed Engl 2017, 56 (3), 733-737.
[4] Kumar, A.; Kinneer, K.; Masterson, L.; Ezeadi, E.; Howard, P.; Wu, H.; Gao, C.; Dimasi, N. Synthesis of a heterotrifunctional linker for the site-specific preparation of antibody-drug conjugates with two distinct warheads. Bioorg Med Chem Lett 2018, 28 (23-24), 3617-3621.
[5] Yamazaki, C. M.; Yamaguchi, A.; Anami, Y.; Xiong, W.; Otani, Y.; Lee, J.; Ueno, N. T.; Zhang, N.; An, Z.; Tsuchikama, K. Antibody-drug conjugates with dual payloads for combating breast tumor heterogeneity and drug resistance. Nat Commun 2021, 12 (1), 3528.
[6] https://www.oncologypipeline.com/apexonco/aacr-2025-preview-surge-dual-payload-conjugates
AACR 2025 preview – a surge in dual-payload conjugates
[7] https://www.aacr.org/meeting/aacr-annual-meeting-2025/abstracts/ AACR Abstracts
[8] https://clinicaltrials.gov/study/NCT06885645
Related Articles:
Development of Current Four Generation ADCs And Next Generation ADCs
Antibody-drug Conjugates (ADCs) - Approvals by FDA/EMA/NMPA/PMDA

